
More detailed information about household income, including what to do if you’re a care leaver, can be found in the household income section. Our FE calculator can help you work out whether you might be able to get EMA based on your household income. Household income must be £23,077 or less if there is more than one young person in the household (this includes you).Household income must be £20,817 or less if you’re the only young person in the household.There are two different levels depending on your family circumstances: Your household income must be below a certain level for you to get EMA. Your course must last at least 10 weeks.You must be studying a minimum of 12 guided hours per week and.You will not be eligible for EMA if you’re receiving an allowance or similar, or being paid for a publicly funded work-based learning programme (such as an apprenticeship) in the UK. Your school or college can tell you if the course you want to do is eligible. What does EMA stand for Your abbreviation search returned 107 meanings. Independent Living Skills courses or, if studying in England, Preparation for Adulthood.Your course should be an academic or vocational course, up to and including Level 3. CourseĪlthough you don't have to study in Wales to get EMA, you must be studying at a school or college involved in the EMA scheme. You should complete the application form and return it to us – we’ll let you know if you’re eligible. If you’re not a UK citizen but you live in Wales, you may still be able to get EMA. The European Medicines Agency (EMA) is a decentralised agency of the European Union (EU) responsible for the scientific evaluation, supervision and safety. If you’re a UK citizen living in Wales, you should be eligible to get EMA. What is an exponential moving average The exponential moving average or EMA gives higher weightage to more recent data points. You must be between 16 and 18 years old on 31 August 2023. cooperate with international partners on the harmonisation of regulatory requirements.Before making your application, there are some things you should check to make sure you’ll be able to get EMA. Beginning with the 2023-24 school year, FTC/FES-EO families will begin to manage their students scholarship through EMA, our new scholarship management.preparing scientific guidelines and regulatory guidance to help pharmaceutical companies prepare marketing authorisation applications for human medicines.providing scientific advice to companies researching and developing new medicines.

In addition, the CHMP and its working parties contribute to the development of medicines and medicine regulation, by: In determining which companies are eligible for SME incentives, EMA applies the EU-definition of micro, small and medium-sized enterprises provided in.
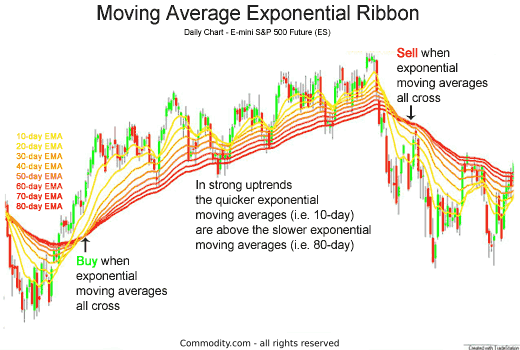
What does the name Ema mean The girl’s name Ema means whole, big, universal and entire (from Old High German ermen/irmin).


For more information, see Referral procedures. Ema is a Spanish, Portuguese or Slavic variant of the name Emma, which is of Old High German origin. The CHMP also evaluates medicines authorised at national level referred to EMA for a harmonised position across the EU. considering the recommendations of the Agency's Pharmacovigilance Risk Assessment Committee on the safety of medicines on the market and when necessary, recommending to the European Commission changes to a medicine's marketing authorisation, or its suspension or withdrawal from the market. EMA: Economic and Market Analysis (various organizations) EMA: Ethnic Minority Achievement (UK education) EMA: Education Maintenance Allowance: EMA: Environmental Media Association (Los Angeles, CA) EMA: Early Middle Ages: EMA: Energy Market Authority (Singapore) EMA: Engine Manufacturers Association: EMA: Employers and Manufacturers.assessing modifications or extensions ('variations') to an existing marketing authorisation.conducting the initial assessment of EU-wide marketing authorisation applications.In the centralised procedure, the CHMP is responsible for: The CHMP plays a vital role in the authorisation of medicines in the European Union (EU).


 0 kommentar(er)
0 kommentar(er)
